What is the difference between copper and electrolytic copper?
Copper is a versatile metal used in various industries due to its excellent electrical conductivity and corrosion resistance. In the field of copper production, two terms appear frequently: copper and electrolytic copper. This article aims to explore the differences between these two forms of copper, clarifying their properties, production processes and equipment technologies.
Copper is a chemical element with the symbol Cu that occurs naturally in ores and is extracted through mining and smelting processes. It is widely used in electrical wiring, plumbing, construction and industrial applications. Copper obtained from mining operations goes through multiple refining steps to achieve purity levels suitable for different applications.
Electrolytic copper, also known as refined copper, is high-purity copper produced through an electrochemical refining process called electrolysis. The process involves the dissolution of an impure copper anode and the subsequent deposition of a pure copper cathode.
Electrolytic copper production process:
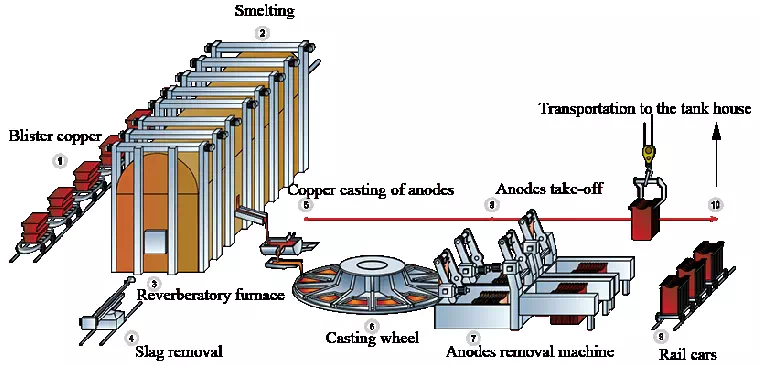
Electrolytic Copper Refining Plant
a) Copper Anode Preparation: Impure copper obtained from mining operations is made into anodes. These anodes often contain impurities such as other metals and elements.
b) Electrolyte solution: An electrolyte solution, usually sulfuric acid, is prepared to facilitate the electrolysis process. The electrolyte solution provides a conductive medium for the flow of electric current.
c) Electrolytic cell: An electrolytic cell consists of an anode and a cathode immersed in an electrolyte solution. The copper anode is connected to the positive terminal (anode) of the power supply, while the pure copper sheet is used as the negative terminal (cathode).
d) Electrolysis process: When an electric current passes through the electrolyte solution, copper ions migrate from the anode to the cathode. When copper ions reach the cathode, they gain electrons and deposit on the surface of the cathode, forming a high-purity copper cathode.
e) Purification and casting: The deposited copper cathodes undergo a further purification process to remove remaining impurities. The purified copper is then melted and cast into ingots or other forms required for commercial use.
Electrolytic copper production equipment technology:

Copper electrolysis workshop
a) Pots: These pots are the heart of the electrolytic copper production process. They consist of an anode compartment and a cathode compartment, separated by a diaphragm or membrane. These electrolytic cells are designed to provide a controlled environment for the electrolysis process, ensuring efficient copper deposition and minimizing contamination.
b) Power supply: A direct current (DC) power supply is used to provide the current required for the electrolysis process. The power supply should provide a stable and adjustable voltage to maintain optimal conditions for copper electrodeposition.
c) Electrolyte Circulation System: An efficient electrolyte circulation system helps maintain the proper chemical composition of the electrolyte, ensuring a consistent and uniform copper deposition process.
d) Filtration and purification device: In order to maintain the quality of the electrolyte solution, a filtration and purification device is used. These units remove impurities, suspended solids and other contaminants, increasing the overall efficiency of the copper cathode production process.
The difference between copper and electrolytic copper:
a) Purity: Copper obtained through traditional mining and smelting processes may contain impurities, whereas copper cathodes have a higher level of purity, typically greater than 99.95%.
b) Conductivity: Due to its high purity and absence of impurities, electrolytic copper exhibits excellent conductivity compared to ordinary copper.
c) Applications: While both forms of copper have applications in various industries, electrolytic copper is particularly suitable for high-precision electrical and electronic components such as printed circuit boards (PCBs) and power transmission systems.
Copper and electrolytic copper are different forms of the same versatile metal. While copper obtained through traditional mining and smelting processes has many uses, electrolytic copper has a higher purity and excellent electrical conductivity. The production process of electrolytic copper involves electrolysis, using special equipment technologies such as electrolytic cells, power supplies and purification devices. Understanding the differences between copper and electrolytic copper can help in selecting the proper form for a specific application, ensuring optimum performance and efficiency across industries.
Recommend products
CONTACT US:
If you have any requirement or suggestion, please fill in the form and send to us, thanks!E-mail:sunymachine@gmail.com | Whatsapp:+8613674945231








